Please click on the tables and figures to enlarge
Remimazolam as a sedative for dental procedures: a case series
A M. Liow1 BDS
N. Akuffo2 BDS
X H. Yeo*3 BDS, MSc Dental Implantology, DipConSed, MFDS RCPS(Glasgow), PgCert in Dental Education, CILT, AKC
S. Clough4 BChD MJDF MSc MSpecCareDent CILT
1Dental Core Trainee, Special Care Dentistry, Royal London Dental Hospital, Turner Street, Whitechapel E1 1FR
2Dental Core Trainee, Special Care Dentistry, Royal London Dental Hospital, Turner Street, Whitechapel E1 1FR
*3Specialty Registrar in Special Care Dentistry, Special Care Dentistry, Royal London Dental Hospital, Turner Street, Whitechapel E1 1FR
4Consultant in Special Care Dentistry, Special Care Dentistry, Royal London Dental Hospital, Turner Street, Whitechapel E1 1FR
*Correspondence to: Dr Xin Hui Yeo
Email: x.yeo@nhs.net
Liow A M, Akuffo N, Yeo X H, Clough S. Remimazolam as a sedative for dental procedures: a case series. SAAD Dig. 2025: 41(I): 28-31
Abstract
Within dentistry, pharmacological support may be required to facilitate the delivery of dental treatment for patients with additional emotional or physical needs. The most common drug of choice for intravenous sedation in dentistry is midazolam due to its efficacy and established safety record. However, the introduction of remimazolam offers a promising alternative with a similar mechanism of action but with a faster onset and recovery, which can be advantageous for patients and service providers.
We present a series of four cases using remimazolam as an intravenous sedative for dental procedures, with varied medical and dental histories and an age range from 19 to 76 years old. We describe the administration regimen, with variation depending on the patient’s age, co-morbidities and individual circumstances. Patients’ pre- and post-operative haemodynamic measures and quality of sedation in these different circumstances were also reported.
Within the limitation of the case series, remimazolam was shown to be an effective sedative agent for dental procedures without any adverse events. Our experience offers promising findings, in particular demonstrating rapid recovery of patients. However, larger scale studies are welcomed in dentistry to further develop the related evidence base.
Key learning points
- Provide readers with an introduction to remimazolam and enhance their understanding through case examples of its use in dentistry
- Help inform readers about the practical aspects of remimazolam use
- Provide readers with an understanding of the professional requirements for remimazolam use.
Introduction
Remimazolam (CNS 7056, PAION UK Ltd., United Kingdom) is an ultra-short-acting intravenous (IV) benzodiazepine, combining some of the properties of two unique drugs already established in sedation and anaesthesia: midazolam and remifentanil. Remimazolam is a midazolam derivative and, like existing benzodiazepines, acts by potentiating GABAA receptors. It differs, however, by the fact it is rapidly hydrolysed by carboxylesterase-1A, producing a pharmacologically inactive metabolite (CNS 7054).1 This inactivation allows for a faster and more predictable recovery profile, compared to midazolam. Remimazolam has a terminal half-life of 0.75 hours whereas midazolam has a terminal half-life of 4.29 hours.2 Remimazolam also has a rapid onset of action which leads to the desired sedation depth in a shorter period of time. Hence, it offers a rapid onset for treatment to commence with a faster recovery, which can be an advantage to both patients and their escorts, as well as service providers.
Remimazolam was approved for marketing by the European Commission on 26 March 2021 and by the UK Medicines and Healthcare products Regulatory Agency (MHRA) in June 2021 for use in adult procedural sedation. However, its use in dentistry was only agreed by the Intercollegiate Advisory Committee for Sedation in Dentistry (IACSD) in January 2023; until now, its use in dentistry has been limited.
Remimazolam use has gained some momentum in medicine, such as sedation for bronchoscopies and colonoscopies, which showed high efficiency and safety as evidenced through systematic review.3-5 Despite a slow start in dentistry, there has been a gradual increase in services obtaining and using remimazolam in the UK. It has been proposed that remimazolam may increase patient throughput and flow, due to its shorter and more predictable recovery and, therefore, could improve cost efficiency.6 A recent cost analysis carried out in Denmark showed economic benefit from the use of remimazolam for patients undergoing bronchoscopies and colonoscopies, compared to procedural sedation with midazolam.7 This is a potential factor to consider in adopting use of remimazolam in dentistry, especially for short clinical procedures.
Additionally, we anticipate that remimazolam could have huge benefits for a range of patients with special care needs, for whom a faster recovery allowing quicker return to baseline cognitive and motor status would be favourable to improve patient safety. It is for this reason that as a special care department we have adopted the use of remimazolam in appropriate clinical situations.
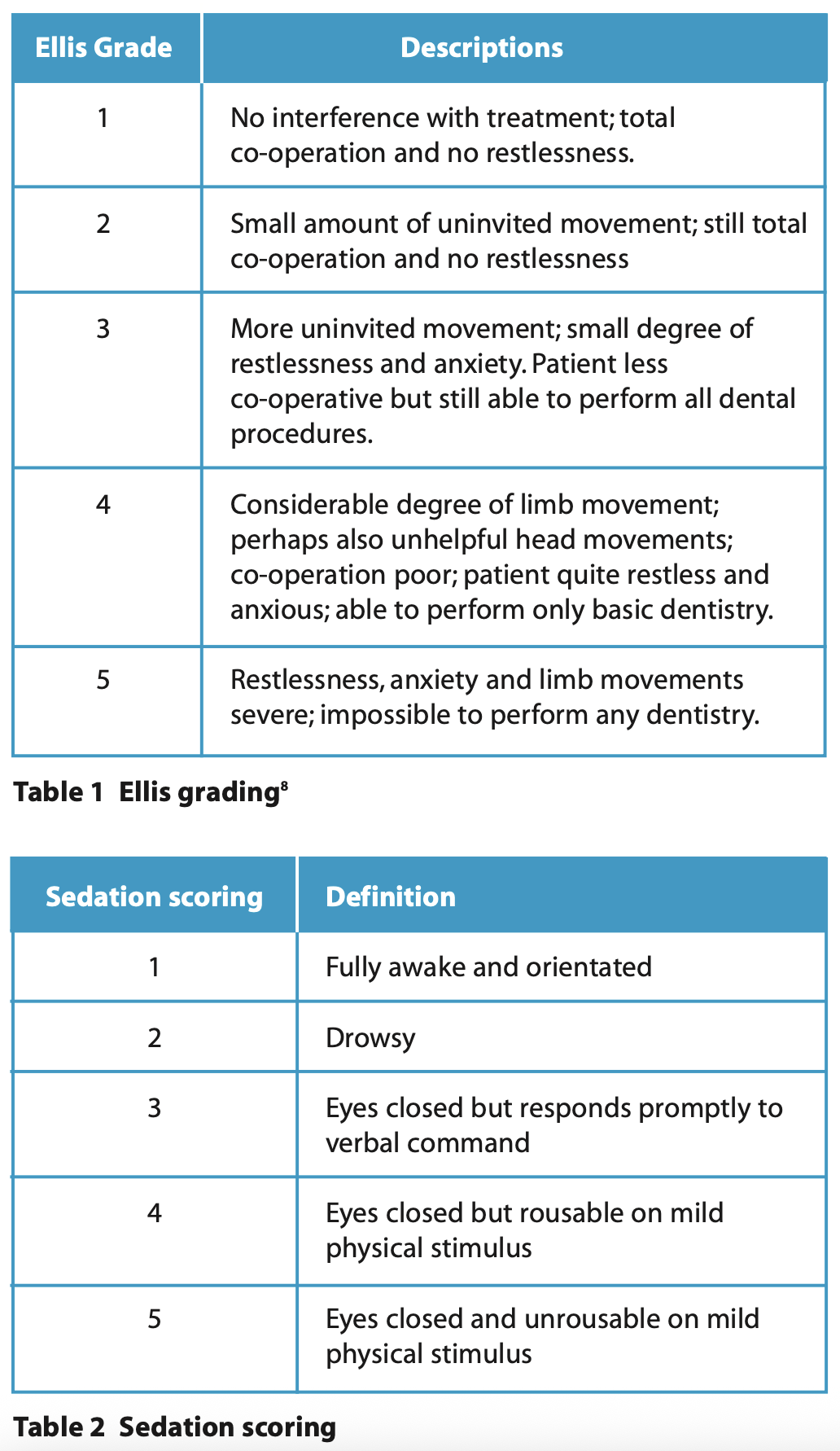
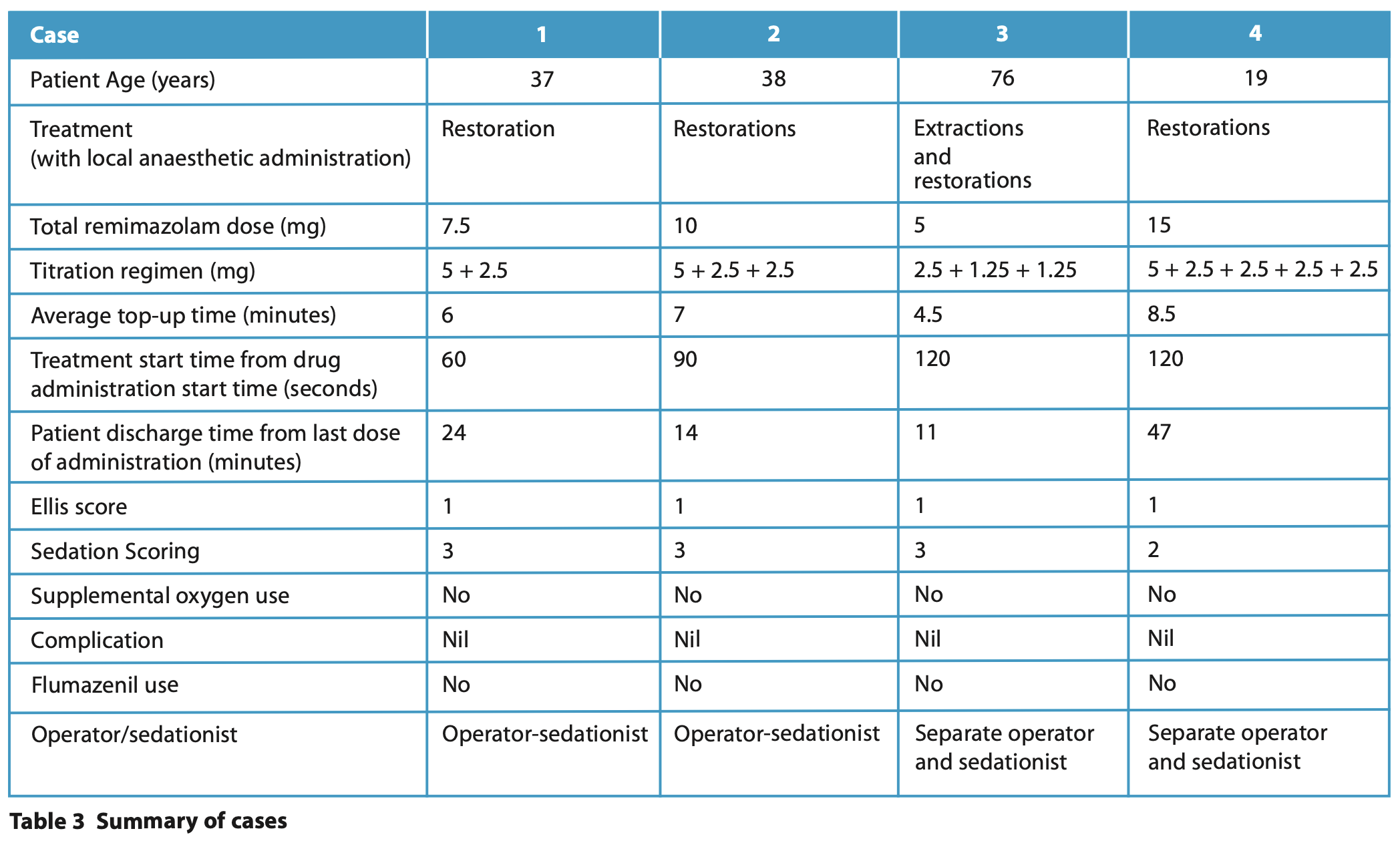
This case series discusses a variety of clinical situations for which remimazolam was used in preference to midazolam as a sedative in the Department of Special Care Dentistry at The Royal London Dental Hospital. We describe the operative conditions using the Ellis scoring system8 and the sedation quality using the sedation scoring, which are summarised for readers in Table 1 and Table 2.
Case 1: a patient with movement disorder
A 37-year-old patient was referred for restoration of teeth under sedation. Medically, the patient had a mixed form of cerebral palsy, with spasticity in her legs and uncontrolled athetoid movements in her upper body. She also experienced palpitations related to anxiety. Her medications included gabapentin and propranolol. She used an electric wheelchair but was able to walk short distances with an unstable gait to transfer in and out of a dental chair. Additionally, she communicated using a digital tablet with a touch-screen facility. She lived independently, with carers visiting twice daily.
Due to the patient’s need to drive her wheelchair safely, as well as communicate accurately via a touch screen, remimazolam was selected as the desired sedative agent, being a shorter acting drug that would enable a faster recovery and safer transfer and discharge for this patient.
She had a pre-operative blood pressure of 123/82 mmHg, heart rate of 96 bpm and O2 saturation was 96%. Titration regimen was as shown in Table 3. Sedation enabled local anaesthetic administration followed by removal and replacement of a defective restoration in the LR6 at this appointment.
Optimal sedation was achieved during this procedure, with no respiratory depression and good operating conditions (Ellis grade 1 and sedation scoring of 3) (Table 2). Additionally, the patient had a swift recovery with no reversal needed. She had a post-operative blood pressure of 122/75 mmHg, heart rate of 99 bpm and 100% oxygen saturation, all similar to her pre-operative measurements. She was discharged 24 minutes after her last dose of sedation, which included an extended conversation regarding her forthcoming appointments and holiday plans.
Case 2: a patient with learning disability and epilepsy
A 38-year-old patient with epilepsy, learning disability, attention deficit hyperactivity disorder (ADHD) and moderate thrombocytopenia attended for multiple restorations under sedation. His medication was sodium valporate. Epilepsy is a condition of the brain which results in recurring seizures. The patient reportedly experienced tonic-clonic seizures characterised by loss of consciousness followed by a period of muscles spasms and absence seizures where typically the person is unaware of their surroundings with minimal uncontrolled movement. Pre- operative blood pressure was 107/68 mmHg, heart rate was 91 bpm and oxygen saturation was 96%.
Remimazolam could be beneficial in patients who have epilepsy and who previously experienced prolonged recovery with midazolam. The pharmacological properties are like midazolam, which is an anti-epileptic, but remimazolam has a quick offset as well as onset which can eliminate the need for the elective use of flumazenil which should be used with caution in this group. In this case, remimazolam allowed for adequate sedation for the duration of the dental treatment. On this occasion, sedation facilitated local anaesthetic administration and restoration of two teeth. No adverse reactions or issues with recovery were noted. He had a post-operative blood pressure of 111/70 mmHg, heart rate of 75 bpm and oxygen saturation of 97%. The patient was discharged 14 minutes after his last dose of remimazolam.
Case 3: an older patient with dementia
A 76-year-old patient with dementia attended for restorations and extractions under IV sedation. Dementia is a brain disorder and is the overarching term used to describe the progressive degeneration of the brain. This patient suffered from a specific type of dementia (Alzheimer's) which in time gradually targets the areas in the brain responsible for thinking and memory resulting in the patient’s inability to conduct tasks independently. She had several co-morbidities which are summarised in Box 2.
The patient’s pre-operative blood pressure was 153/79 mmHg, heart rate was 119 bpm and oxygen saturation was 99%.
In view of the patient’s age and medical history, an adjusted administration regimen was used (Table 3). The excretion and metabolism of remimazolam are not significantly affected by renal or hepatic function, which can be reduced in older people. The patient had some mobility issues thus using a sedative drug with a shorter recovery time meant that the patient could self-transfer back to her wheelchair shortly after completion of treatment whilst also enabling her to return to cognitive baseline swiftly. This would aid the escort in transporting the patient home safely and reduce the risk of falls.
The patient was adequately sedated for the duration of the dental treatment, enabling administration of local anaesthetic followed by extraction of two teeth, LR7 and LR5 and two restorations, in UL3 and LL3. No adverse reactions or issues with recovery were noted. She had a post-operative blood pressure of 115/65 mmHg and oxygen saturation of 98%. The patient was fit for discharge 11 minutes after her last dose of sedation.
Case 4: a patient with failed sedation with midazolam
A 19-year-old patient attended for multiple restorations under IV sedation. This patient had a history of psychological trauma as a child and dental phobia. Her only medication was the combined oral contraceptive pill. Dental phobia is an unproportional fear response to dentistry. These fears may originate from past negative experiences, fear of pain or general anxiety about unknown procedures. In this case, it was compounded by her state of mental health and the association of having dental treatment in a vulnerable position.
Her pre-operative blood pressure was 126/80 mmHg, heart rate was 68 bpm and oxygen saturation was 100%.
Remimazolam was beneficial in this case by allowing better control of sedation depth. The patient had previously demonstrated disinhibition when sedated with midazolam, where she became emotional and less co-operative in a deeper, prolonged state of sedation. Remimazolam, with its quick onset and offset, allowed the team to titrate the drug to the patient’s response in a way that it provided sufficient anxiolysis, but she still felt that she retained some form of control throughout the whole procedure and was reassured that this would wear off quickly. The patient self-reported a better overall experience with remimazolam compared to midazolam following the appointments. On this occasion, she had local anaesthetic administration followed by restorations in LL6 and LL7. The patient had a post-operative blood pressure of 137/69 mmHg, heart rate of 66 bpm and 100% oxygen saturation. The patient was discharged 47 minutes after her last dose of sedation.
Discussion
As these cases demonstrate, remimazolam can be used as an effective sedative drug for patients with a variety of treatment needs and medical backgrounds to facilitate safe delivery of dental care. There is an initial learning curve involved in adopting the use of remimazolam in sedation services which can be addressed by training courses and supervised clinical practice. Teams using remimazolam should adhere to the same clinical standards and training required for midazolam.9 Clinicians should be able to demonstrate evidence of related continuing professional development courses on top of fulfilling annual immediate life support requirements.
Remimazolam is being used as a single drug in dentistry at this stage. Drug manufacturing guidance recommends a bolus dose of 7 mg and states that the patient should be monitored throughout by a separate healthcare professional who is not involved in carrying out the dental procedure, and whose sole task is to monitor the patient.10 However, IASCD guidance states that remimazolam is classed as a basic technique and could be carried out by an operator-sedationist.9 The cases described in this case series were a mixture of operator-sedationist and separate operator and sedationist techniques, depending on the complexity of individual patient needs. In our service, a lower than manufacturer-recommended bolus dose of 5 mg followed by 2.5 mg increments were used for the titration regimen. For older people or medically complex patients, such as case three, an even lower dosing regimen was used (2.5 mg followed by 1.25 mg increments).
Remimazolam has a shorter recovery and hence a shorter time to alertness and restoration of neuromotor function,3 so it is ideal for patients with mobility issues or patients with cognitive impairment, for example, learning disability or dementia where prolonged recovery poses a challenge to management. Across the four cases, we had an average discharge time from last remimazolam dose of 23.75 minutes, with the shortest time being only 11 minutes. This highlights the benefit of rapid recovery that remimazolam can provide. Unlike midazolam, it undergoes organ-independent metabolism and elimination, making it suitable for patients with renal or hepatic failure, although they are highlighted as special populations in the product literature.11 Like midazolam, the availability of an antagonist (flumazenil) adds to its safety in case of over-sedation. The most frequent adverse reactions in patients with intravenous remimazolam are hypotension (37.2%), respiratory depression (13.1%) and bradycardia (6.8%).9 However, none of these adverse outcomes were detected in these cases.
Remimazolam is only licensed for use in patients over the age of 18. This case series included two older patients and three ASA III patients, but no ASA IV patients or patients with low body weight <50 kg - it is sensible to consider a lower initial dose and slower titration regimen for these groups of patients. It is interesting to note that when the adjusted titration regimen was used for case three, the average top-up intervals were shorter compared to a standard regimen, meaning more frequent top-ups were required throughout treatment. In such circumstances, a separate sedationist can sometimes be beneficial to ensure a smooth sedation experience for the patient and operator. An infusion pump with a pre-set individualised infusion rate might be worth considering for remimazolam sedation in the future.
The limitations of this case series include the small sample size and limited timeframe in one clinical setting (secondary care). It would also be useful to demonstrate patient-reported outcome measures (PROMs) and patient-reported experience measures (PREMs) in future scientific reports.
Conclusion
Remimazolam offers an alternative to midazolam, but with quick onset and recovery. This case series showed some encouraging data regarding the effectiveness of remimazolam in dental sedation with case selection, with no adverse events reported. The overall sedation success with remimazolam looks promising for both restorative and surgical procedures in our setting. Certain aspects of this drug acquisition require further examination, such as cost-effectiveness, before its widespread acceptance and usage.
Acknowledgements
Thank you to all staff in the Department of Special Care Dentistry and Rebekah Mar-Watts (scheduler) at The Royal London Dental Hospital for their continued support and willingness to work in a new way to improve care for our patients.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
1. Kilpatrick G J, McIntyre M S, Cox R F, et al. CNS 7056: a novel ultra-short-acting Benzodiazepine. Anesthesiology. 2007; 107: 60-66. doi: 10.1097/01.anes.0000267503.85085.c0.
2. Kim K M. Remimazolam: pharmacological characteristics and clinical applications in anesthesiology. Anesth Pain Med (Seoul). 2022; 17: 1-11. doi: 10.17085/apm.21115.
3. Noor N, Legendre R, Cloutet A, Chitneni A, Varrassi G and Kaye A D. A comprehensive review of remimazolam for sedation. Health Psychol Res. 2021; 9: 24514. doi: 10.52965/001c.24514.
4. Oka S, Satomi H, Sekino R, et al. Sedation outcomes for remimazolam, a new benzodiazepine. J Oral Sci. 2021; 63: 209-211. doi: 10.2334/josnusd.21-0051.
5. Zhu X, Wang H, Yuan S, et al. Efficacy and Safety of Remimazolam in Endoscopic Sedation—A Systematic Review and Meta-Analysis. Front Med (Lausanne). 2021; 8: 655042. doi: 10.3389/fmed.2021.655042.
6. Lee A, Shirley M. Remimazolam: A Review in Procedural Sedation. Drugs. 2021; 81: 1193-1201. doi: 10.1007/s40265-021-01544-8.
7. Pedersen M H, Danø A, Englev E, Kattenhøj L, Munk E. Economic benefits of remimazolam compared to midazolam and propofol for procedural sedation in colonoscopies and bronchoscopies. Curr Med Res Opin. 2023; 39: 691-699. doi: 10.1080/03007995.2023.2196198.
8. Ellis S. Response to intravenous midazolam sedation in general dental practice. Br Dent J. 1996; 180: 417-420. doi: 10.1038/sj.bdj.4809108.
9. Intercollegiate Advisory Committee for Sedation in Dentistry. Remimazolam for intravenous conscious sedation for dental procedures: IACSD standard on clinical use and training. 2023. Online information available at https://www.rcseng.ac.uk/-/media/FDS/IACSD/IACSD-remimazolam-Statement-130623.pdf/media/FDS/IACSD/IACSD-remimazolam-Statement-130623.pdf (Accessed June 2024)
10. European Medicines Agency. Remimazolam Product Information. 2021. Online information available at https://www.ema.europa.eu/en/documents/product-information/byfavo-epar-product-information_en.pdfhttps://www.ema.europa.eu/en/documents/product-information/byfavo-epar-product-information_en.pdf (Accessed June 2024)
11. Stöhr T, Colin PJ, Ossig J, et al. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth. 2021; 127: 415-423 doi: 10.1016/j.bja.2021.05.027.