Please click on the tables and figures to enlarge
A service evaluation of the use of IV sedation with remimazolam for adults with an acquired brain injury
M. Doshi*1, BDS (Hons) MSc (Spcsed)
R. Prasad1, MJDF MSc (SpcSed) MSCD
D. Reilly BDS Dip(sed)1
1Consultant in Special Care Dentistry, Royal Hospital for Neuro-disability, West Hill, Putney, London, SW15 3SW
*Correspondence to: M. Doshi
Email: mili.doshi@nhs.net
Doshi M, Prasad R, Reilly D. A service evaluation of the use of IV sedation with remimazolam for adults with an acquired brain injury. SAAD Dig. 2025: 41(I): 22-27
Abstract
Introduction
Remimazolam is an ultra-short-acting benzodiazepine that is an alternative to midazolam for dental sedation. Limited data exist on its use in dentistry for patients with medical complexities.
Methods
This study prospectively evaluated all patients receiving remimazolam for intravenous (IV) sedation by the Royal Hospital for Neuro-disability dental team over a period of six months.
Results
46 episodes of sedation with remimazolam were undertaken on 33 patients with an American Society of Anaesthesiologists (ASA) grade of 3. The mean dose of remimazolam was 11 mg. Total co-operation, with and without small amounts of uninvited movements, was documented to be 70%. The discharge time from the last dose of remimazolam ranged from 10-50 minutes. Four patients required supplemental oxygen or airway manoeuvres.
Conclusion
The data collected indicates that remimazolam may offer a safe and effective alternative to midazolam in this patient cohort.
Key learning points
- Remimazolam has a shorter half-life compared to midazolam, potentially benefiting patients with medical multimorbidity
- Remimazolam has good muscle relaxation properties, which are beneficial for patients with brain injuries whose mouth opening is often limited
- More studies are needed to assess the patient-centred benefits and cost implications of using remimazolam for dental conscious sedation.
Introduction
Dentist-led conscious sedation for people with disabilities is a safe and effective way to provide care in a community and hospital setting.1 Patients with an acquired traumatic injury (for example, as a result of a road traffic accident), non-traumatic (eg stroke), or inherited brain injury (eg Huntington’s disease) may not be able to accept dental examinations or treatment as they once did for several reasons, including oral hypersensitivity, limited mouth opening, challenging behaviours, involuntary movement and increased anxiety.2 It is important that these patients can still access dental care, including comparable, appropriate pain and anxiety management techniques, in an equitable manner compared to the general population.3
The Royal Hospital for Neuro-disability (RHN) is a specialised care facility in Putney, southwest London. It provides care for 250 patients with acquired or inherited brain injuries, divided into long-term residents and patients admitted for shorter rehabilitation periods. At the time of writing, 74% are fed via an enteral route, and 28% have a tracheostomy tube in situ.4
A dental clinic is commissioned to provide NHS care to all residents within the hospital grounds. 98% of patients use a wheelchair, so all treatment is undertaken on a wheelchair recliner. Residents often have significant disorders of consciousness (DOC), which is a state of prolonged altered consciousness, categorised into coma, vegetative state, or minimally conscious state, based on neurobehavioral function.5 These patients will appear awake or asleep but have minimal or no level of consciousness and are, therefore, unable to cooperate with mouth opening.
Dentist-led intravenous (IV) sedation with midazolam has been used as an adjunct to facilitate dental care at RHN since 2004. Midazolam has an excellent safety record and proven efficacy, but the main undesirable side effects are respiratory depression and a relatively prolonged recovery time.1 This may be of particular concern in specific patient groups, such as those with compromised respiratory functions or in a minimally conscious state. This can be the case with many of the patients, who tend to be medically complex with an American Association of Anaesthetists (ASA) physical status classification of 3 and often have relatively low baseline oxygen saturations compared to the general population. A longer sedation recovery time in this cohort of patients has been evidenced through the use of bispectral index monitoring, which measures the level of consciousness.6 Prolonged recovery may prevent patients from accessing other activities scheduled for the day, including physiotherapy, occupational therapy, psychology sessions, speech and language therapies, or visits from friends and family. Missing these interactions may have a negative impact on patients’ physical and mental wellbeing.
Remimazolam, an ultra-short-acting benzodiazepine, has been licensed for procedural sedation in the UK since 2021 and is supported by the Intercollegiate Advisory Committee on Sedation in Dentistry (IACSD)7 with a position statement. It offers a rapid onset of action, minimal effect on respiration and circulation and a low incidence of adverse reactions.8 The onset time for remimazolam is 1-2 minutes compared to 3 for midazolam, and recovery time is 10-40 minutes compared to 20-80.9,10 Remimazolam is metabolised by tissue esterases in the blood to its inactive metabolite, carboxylic acid, which allows for rapid removal of the drug, explaining the fast recovery time compared to midazolam, which is metabolised by the liver into active metabolites.11
At the time of writing, there is no published literature on using remimazolam for dental sedation in ASA 3 patients. Growing research supports its use in sedation for more medically complex patients for medical procedures; a study on colonoscopy procedures in ASA 3 patients found faster sedation and recovery times and few side effects.12 The main potential advantage of using remimazolam in our patient cohort is the faster recovery time compared with midazolam. This faster recovery time suggests two possible significant advantages over midazolam:
- A shorter period of sedation-related central nervous system depression may reduce the likelihood of aspiration, a significant risk for many patients with a brain injury
- It allows patients to participate in other activities on the day of sedation, which offer physical and mental benefits.
The aim of this service evaluation is to identify the potential benefits of using remimazolam in this patient cohort by:
- Assessing operating conditions (Ellis scoring)13
- Measuring the rate of sedation complications
- Assessing the time taken for recovery.
Method
This study prospectively evaluated all patients receiving remimazolam for IV sedation from 1 August 2023 to 26 April 2024. As a new drug with limited research in this patient group, the evaluation was agreed upon with the hospital's Drug and Therapeutics Committee and approved by the audit lead.
Initially, remimazolam sedation was performed by a separate operator and sedationist. As proficiency with the technique improved, a single operator-sedationist provided the sedation. A sedation-trained dental nurse assisted in all cases.
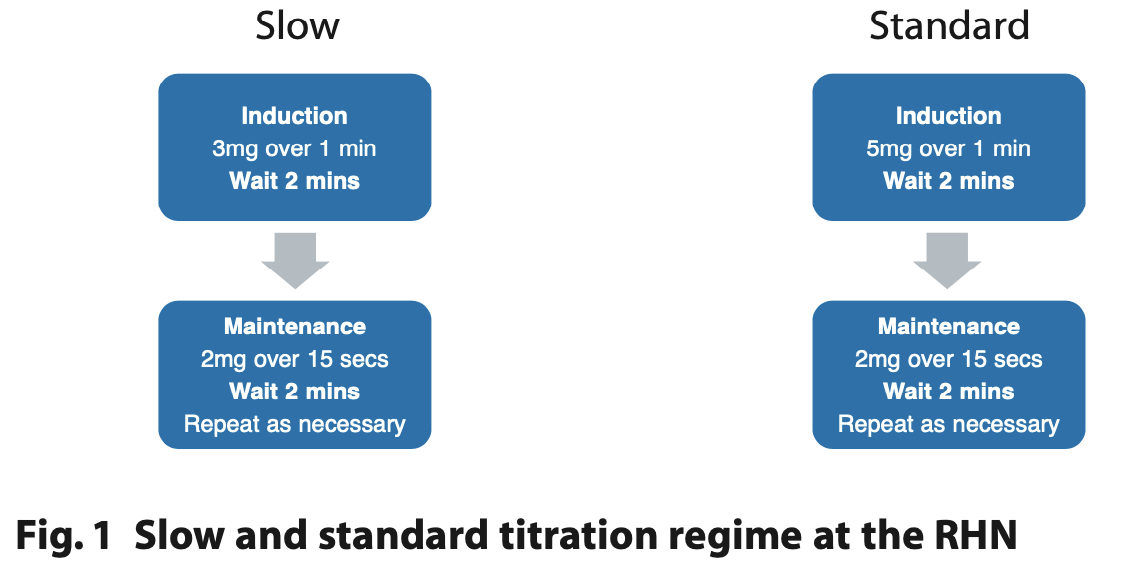
Remimazolam (20 mg per vial) was reconstituted with 20 ml of saline in a 20 ml syringe to be administered 1 mg/1 ml for ease of titration. Depending on the medical status, it was titrated as a slow or standard regime adapted from the drug manufacturers' guidance (Figure 1).14
After treatment, patients were monitored in the clinic and discharged into the care of a ward nurse. Patients were reviewed in the afternoon in their ward.
The following data were collected, and recorded on an Excel spreadsheet for each sedation case.
- Age
- Primary cause of brain injury
- ASA grade
- Baseline oxygen saturation and pulse
- Remimazolam titration regimen (slow / standard)
- Time taken to get to endpoint (time at which dental assessment or treatment started)
- Total dose of remimazolam
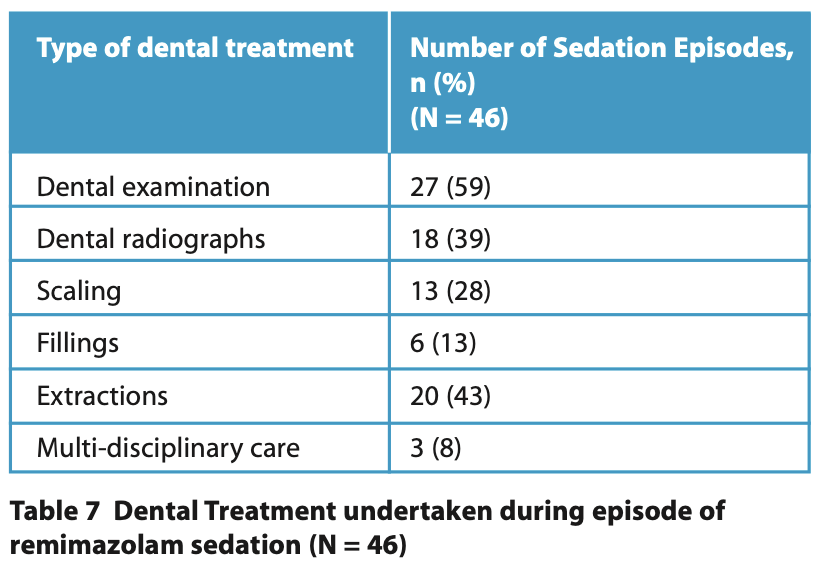
- Dental treatment undertaken
- Sedation score
- Dedicated operator and sedationist or operator-sedationist
- Recovery time after the last increment of remimazolam
- Complications
- Use of flumazenil
- Comments of the sedation team.
Results
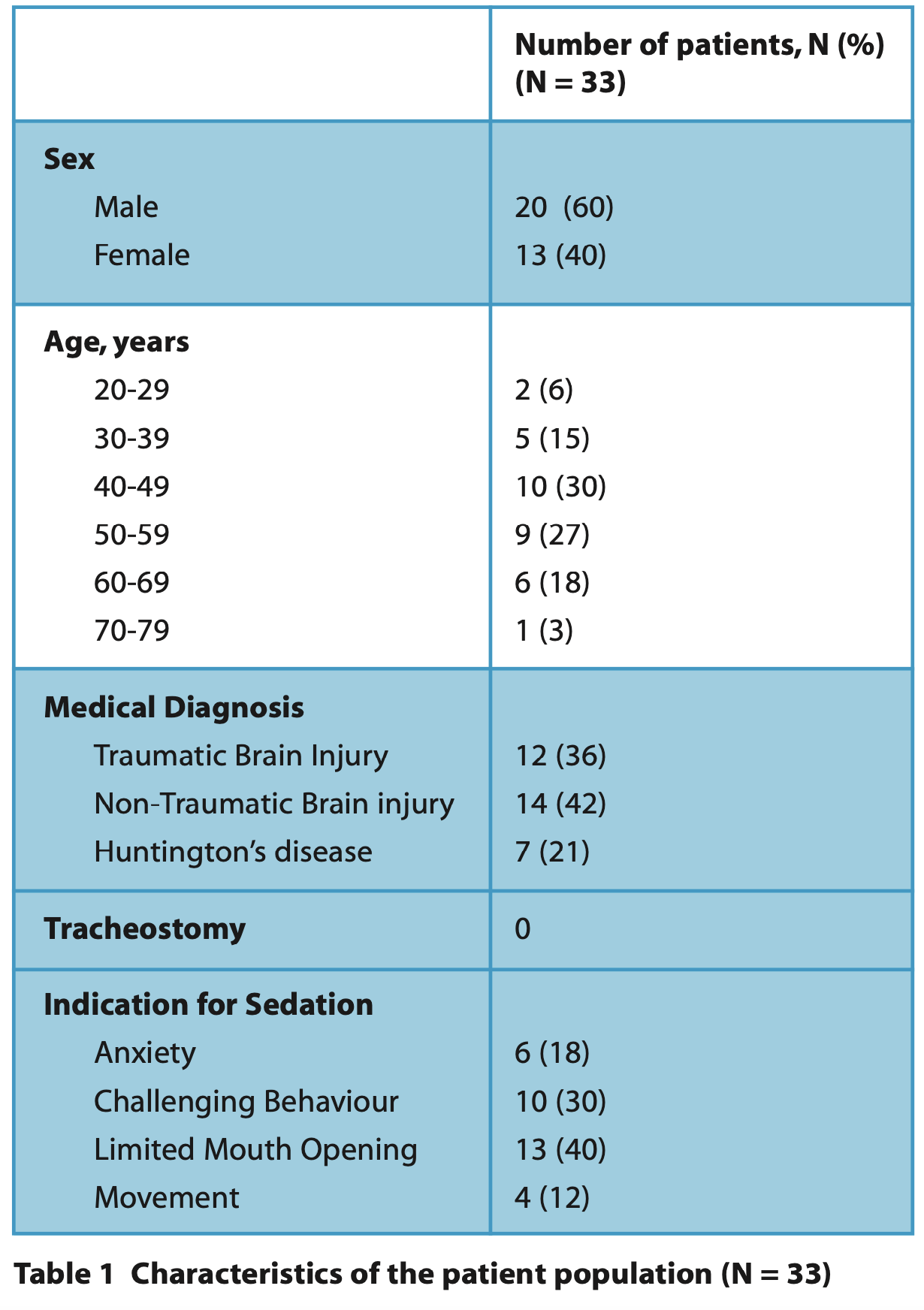
Patient characteristics (Table 1)
Thirty-three patients were sedated between 1 August 2023 to 26 April 2024 with some individuals (n = 10, 30%) having more than one instance of sedation, giving a total of 46 episodes. The patients' ages ranged between 21 and 75 years, with a mean of 49 years and included 13 females and 20 males. All patients were classified as ASA 3. The most-cited indication for sedation was limited mouth opening (n = 13, 39 %) and challenging behaviour (n = 10, 30%). Table 1 summarises the characteristics of the patients included in this service evaluation.
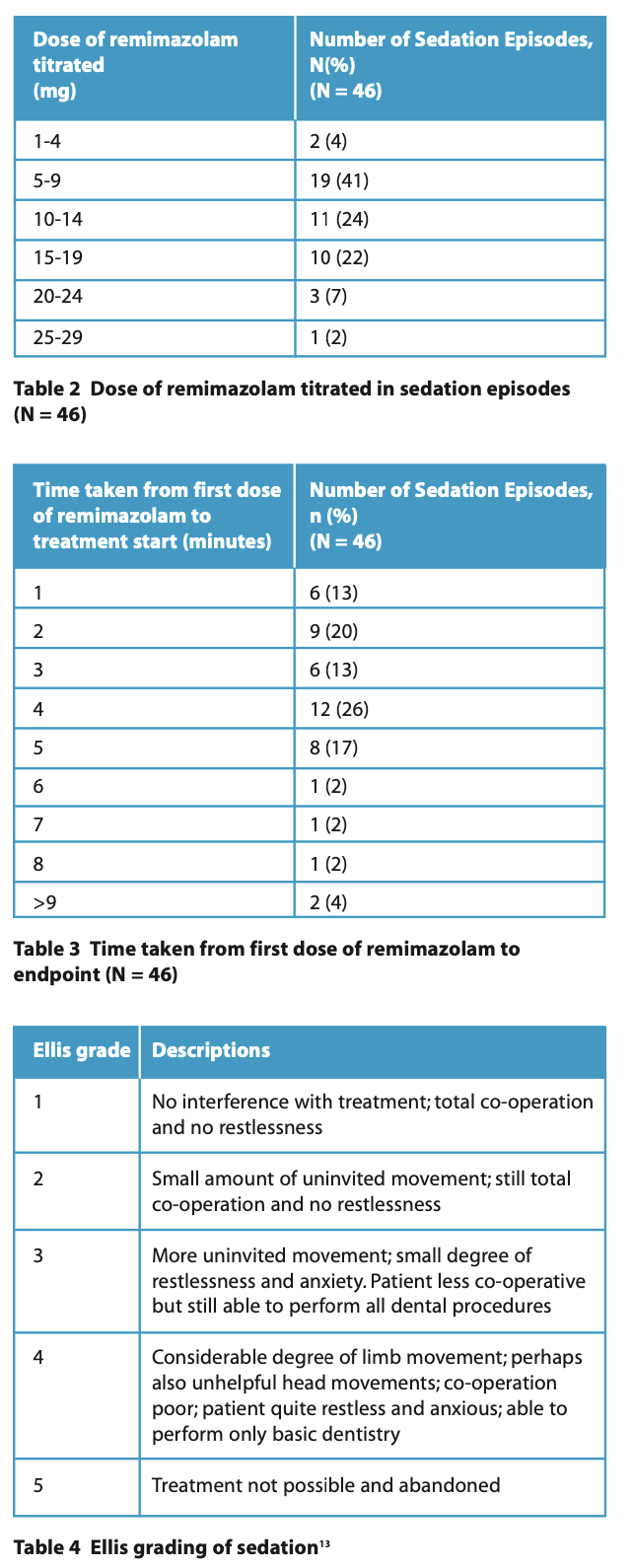
Sedation (Tables 2, 3 and 4)
In 50% of cases, sedation was performed by a separate operator and sedationist. A slow titration regimen was undertaken in 29 (82%) episodes of sedation. The baseline oxygen saturation ranged between 93% and 100%, with a mean of 97%.
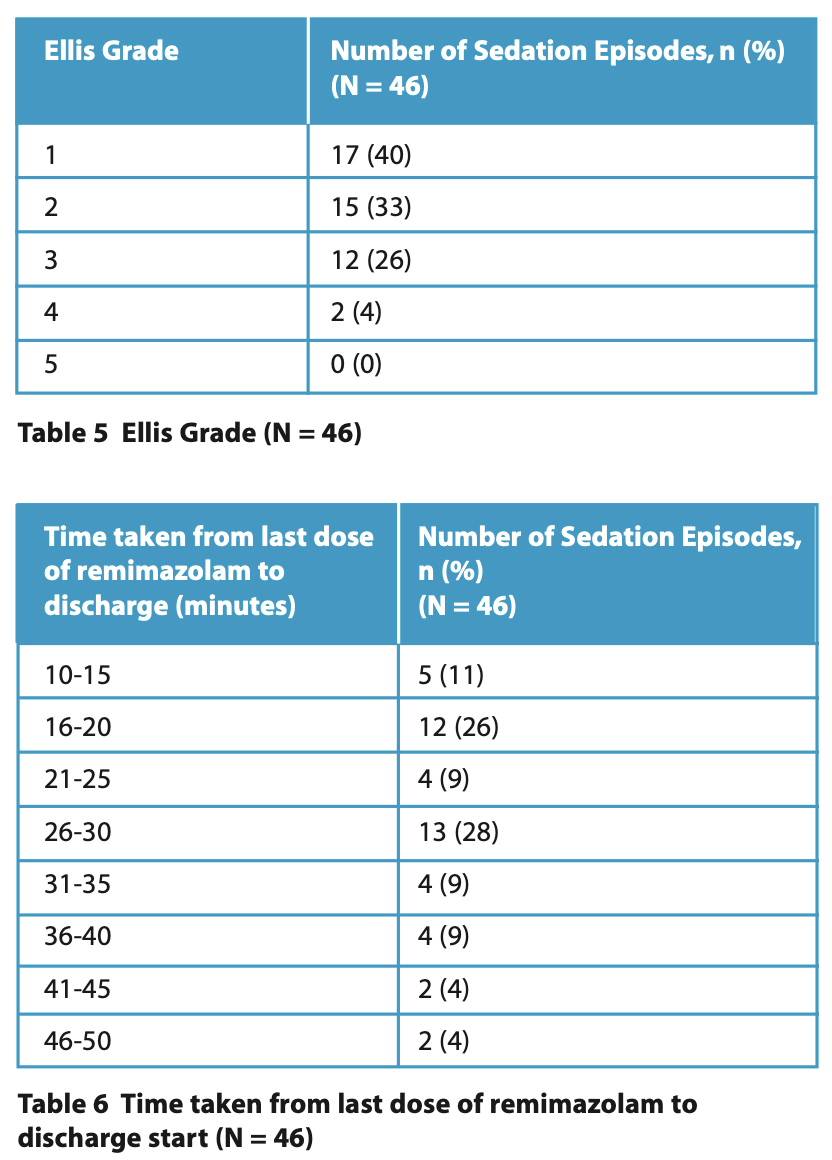
Tables 2 and 3 summarise the total dose of remimazolam titrated during each sedation episode and the time taken to achieve the endpoint. The lowest total amount of remimazolam given was 3 mg, the highest was 25 mg, and the mean was 11 mg. In most instances, dental treatment commenced 4 minutes after the first dose of remimazolam. Total co-operation, with and without small amounts of uninvited movements (Ellis Grade 1 and 2), was documented in 32 (70%) of cases (Table 5). The discharge time from the last dose of remimazolam ranged from 10 to 50 minutes, with a mean of 27 minutes.
Dental examination was the most frequent dental treatment (n = 27, 59%). Other treatments included radiographs, scaling, fillings, extractions and multidisciplinary care, such as botulinum toxin administration and muscle tone assessment (Table 7).
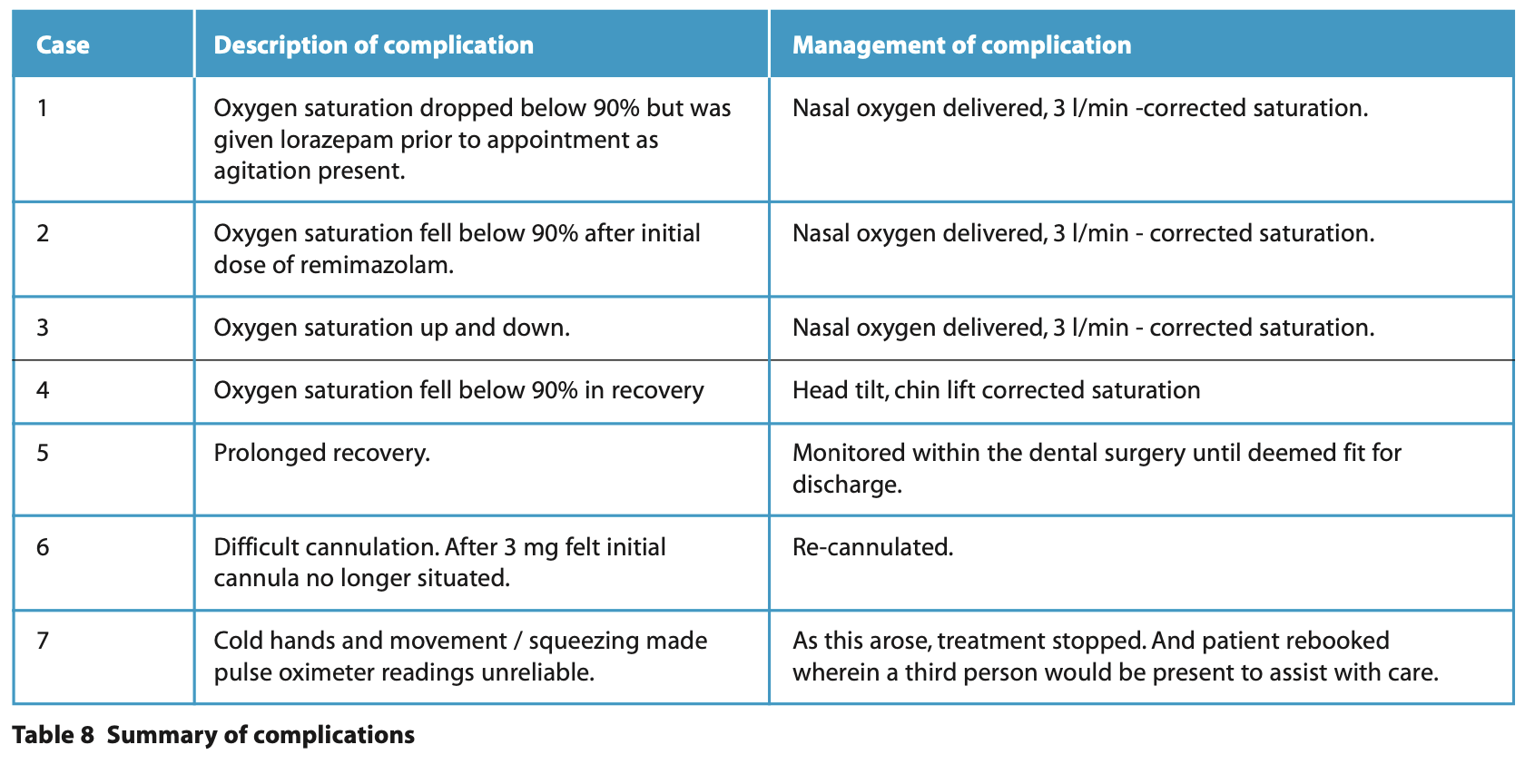
Complications
From the 46 episodes of sedation, minor complications were documented in seven (15%) cases, Table 8 summarises the complications and their management. Flumazenil was not required in any cases to reverse the effects of remimazolam.
Discussion
The evaluation indicates that remimazolam may offer a safe and effective alternative to midazolam in this patient cohort. In this evaluation, limited mouth opening was the most common indication for sedation (40%). Limited mouth opening in patients with a brain injury is often due to changes in muscle tone, muscle spasms or hypersensitivity. Benzodiazepines such as remimazolam, in addition to their sedative properties, also produce muscle relaxation, which is needed in this patient group. For patients who resist examination, a decision must be made to monitor them actively or use a pharmacological technique, such as sedation or general anaesthesia, to undertake dental care. In this patient group, especially those with a DOC, reliance on signs of pain / infection is difficult, and the oral assessment is often challenging. There is an indication for examination under sedation at intervals based on oral health risk factors and medical suitability for sedation. A sedative with a rapid recovery is of benefit for short procedures such as examinations and radiographs.
Midazolam has been the first-line drug for dentist-led IV sedation for 30 years; however, in the last five years, there has been an increasing interest in remimazolam. Research on remimazolam has indicated several benefits compared to midazolam, including a faster uptake of sedation, minimal respiratory depression and quicker recovery. People with a profound brain injury tend to have increased medical comorbidities, including neurological, endocrine, cardiovascular and respiratory conditions and polypharmacy. All patients in this evaluation were ASA 3, representing the medical complexities of the patient population. Midazolam, when titrated slowly and carefully by experienced clinicians, has been found to be safe and effective in this group; however, it can take patients a relatively long time to recover and it is difficult to assess when they have returned to their baseline, especially in those with a DOC.6
A range of doses of remimazolam were administered, with low doses for examinations and higher doses for longer procedures. In most cases, the remimazolam was given a slow titration due to the complexity of the patients. As remimazolam has a shorter half-life than midazolam, increments throughout the procedure can be given to maintain the level of sedation, creating a longer sedation window for treatment without significantly increasing recovery time. When sedating with midazolam, further increments can also be given, but the relatively long half-life can extend the recovery time beyond what is desirable or practical.
The onset of remimazolam sedation was relatively fast and treatment could be started, in some cases, within a minute. However, due to patient-related factors, including the absence of verbal communication and low baseline levels of consciousness, it was sometimes difficult to determine when the patient was at their peak level of sedation. This may be why the endpoint was sometimes clinically judged to take more than five minutes.
The Ellis scale13 was used in this study, as opposed to other sedation scoring methods, which assess the levels of drowsiness and responsiveness to command. Eliciting these responses is often not possible in patients with a brain injury, especially those with a DOC. Only two patients were scored as Ellis 4 when only a limited examination was possible, indicating that remimazolam was an effective sedative in this evaluation.
Sedation recovery times varied with an average of 27 minutes. Remimazolam has a shorter elimination half-life (37 to 53 minutes) than midazolam (1.5 to 2 hours),9 and recovery times have been found to be faster in dental studies.10 The perception of the treating clinicians was that the patients recovered faster with remimazolam than with midazolam. Upon reviewing patients in their wards in the afternoon, those who could usually engage in therapies could still do so. Often, patients who have IV midazolam tend to go back to bed for the rest of the day. This prevents them from participating in other activities and family visits, which are essential for their rehabilitation and mental health.
Minor complications were documented in 17% of remimazolam cases, and 8% were for oxygen desaturation, corrected by airway manoeuvres or supplementary oxygen. Dental studies using remimazolam have found a low rate of complications and minimal respiratory depression.15
One barrier to using remimazolam is the increased cost. At the time of writing, in a hospital, the cost of remimazolam, including VAT, was £22.50 per 20mg vial, and for midazolam £0.21 per 5mg/mL vial. However, as discussed, it can be argued that the benefit to patients outweighs the higher cost implications, especially in this medically-complex patient cohort. A faster recovery time also creates additional clinical capacity.
From a practical perspective, there are differences between the use of remimazolam and midazolam. Remimazolam must be reconstituted before administration. Doses may need to be given more frequently due to the shorter half-life. All clinicians providing sedation were specialists / consultants in special care dentistry who were experienced in conscious sedation and in treating patients with profound brain injuries. It was the experience of these clinicians that it was useful to have a separate sedationist and operator to start with. However, with appropriate experience, it was felt that it could also be used as a single operator-sedation technique for relatively simple procedures. All clinicians were in agreement that remimazolam was a useful addition for sedation, particularly for undertaking short procedures for patients with acquired brain injury and for muscle relaxation.
This service evaluation shows promising, early findings in a field with very limited research. More studies of the use of remimazolam in dentistry, including for patients with disabilities, are needed to justify the patient benefits versus the cost.
Conclusion
Whilst midazolam has been and continues to be a safe and effective IV sedation drug, the introduction of remimazolam does bring potential benefits, including a faster recovery time. In patients with acquired and inherited brain injuries, remimazolam produced adequate levels of sedation and muscle relaxation to allow dental assessment and treatment and patients recovered faster. This evaluation shows that remimazolam is a promising new drug in dental sedation, and more data collection is needed to increase the amount of evidence in this field.
Conflicts of Interest
The authors declare no conflict of interests.
Acknowledgements
The authors would like to thank the Royal Hospital for Neurodisability for supporting the introduction of remimazolam into the service and Dental Nurse Manager, Louise Gallagher, for supporting undertaking treatment.
References
1. Shehabi Z, Flood C, Matthew L. Midazolam use for dental conscious sedation: how safe are we? Br Dent J. 2018 Jan; 224: 98–104.
2. Manley M C G, Doshi M. The importance of oral health and the value of dental care in the process of rehabilitation for people with complex neuro-disability. Disabil Rehabil. 2021 Jan 16; 43: 297–8.
3. Manley M C G, Lane H L, Doshi M. Dental disadvantage for people with disability: a potential solution for a problematic area of care. Disabil Soc. 2021 Aug 9; 36: 1197–202.
4. The Royal Hospital for Neurodisability [Internet]. Available from: https://www.rhn.org.uk/ (Accessed 18 June 2024)
5. Royal College of Physicians Prolonged disorders of consciousness following sudden onset brain injury: national clinical guidelines 2020. Online information available at: https://www.rcp.ac.uk/media/ptcoggi5/pdoc-guidelines_final_online_0_0.pdf (Accessed 18 June 2024)
6. Keddie M, Shah P, Vincer H, Doshi M. An investigation of bispectral index monitoring when providing intravenous sedation for adults with severe cognitive disability. SAAD Dig. 2024; 40:23–7.
7. Intercollegiate Advisory Committee for Sedation in Dentistry. Royal College of Surgeons of England. http://www.rcseng.ac.uk/fds/committees/intercollegiateadvisory-committee-for-sedation-in-dentistry V1.1 2020. (Accessed 8 October 2024)
8. Hu Q, Liu X, Wen C, Li D, Lei X. Remimazolam: An Updated Review of a New Sedative and Anaesthetic. Drug Des Devel Ther. 202; 16: 3957–74.
9. Kim K M. Remimazolam: pharmacological characteristics and clinical applications in anesthesiology. Anesth Pain Med. 2022 Jan 31; 17: 1–11.
10. Li X, Tian M, Deng Y, She T, Li K. Advantages of Sedation With Remimazolam Compared to Midazolam for the Removal of Impacted Tooth in Patients With Dental Anxiety. J Oral Maxillofac Surg. 2023;81:536–45.
11. Sneyd J R. Remimazolam – current status, opportunities and challenges. Anesthesiol Perioper Sci. 2023; 1: 2-10
12. Pantos M M, Kennedy D R, Nemec E C. Remimazolam: A Novel Option for Procedural Sedation in High Risk Patients. J Pharm Pract. 2023; 36 :149–54.
13. Ellis S. Response to intravenous midazolam sedation in general dental practice. Br Dent J. 1996; 180: 417–20.
14. SUMMARY OF PRODUCT CHARACTERISTICS [Byfavo]. Available from: https://www.ema.europa.eu/en/documents/product-information/byfavo-epar-product-information_en.pdf. (Accessed 29 Jun 2024).
15. Oue K, Oda A, Shimizu Y, Takahashi T, Kamio H, Sasaki U, et al. Efficacy and safety of remimazolam besilate for sedation in outpatients undergoing impacted third molar extraction: a prospective exploratory study. BMC Oral Health. 2023; 23: 774.