Please click on the tables and figures to enlarge
An investigation of bispectral index monitoring when providing intravenous sedation for adults with severe cognitive disability
M. Keddie BDS Hons, MJDF RCS Eng, Dip SCD RCS Ed*1
H. Vincer BDS, MFDS RCPS(Glasg)2
P. Shah BDS, MFDS RCS Eng, MSc SCD SED3
M. Doshi BDS Hons, MFDS RCS Eng, MSc SCD SED4
1Senior Dental Officer, Surrey and Sussex Healthcare NHS Trust, East Surrey Hospital, Canada Avenue, Redhill, RH1 5RH
2Dental Officer, Kent Community Health NHS Foundation Trust, The Rainbow Centre, Great Chart Bypass, Ashford, Kent, TN23 4RR
3Consultant in Special Care Dentistry, Kent Community Health NHS Foundation Trust, Canterbury Health Centre, 26 Old Dover Road, Canterbury, Kent, CT1 3JH
4Consultant in Special Care Dentistry, Royal Hospital for Neuro-disability, West Hill, Putney, London, SW15 3SW
*Correspondence to Dr Meg Keddie
Email: megkeddie@nhs.net
Keddie M, Vincer H, Shah P, Doshi M. An investigation of bispectral index monitoring when providing intravenous sedation for adults with severe cognitive disability. SAAD Dig. 2024: 40(1): 23-27
Abstract
Introduction
When providing intravenous sedation for patients with severe disabilities who have limited verbal communication it can sometimes be challenging to identify when sedation endpoint is reached. Bispectral index monitoring can provide an objective measure of the depth of sedation.
Aim
This study aims to assess the feasibility of bispectral index monitoring in adults with cognitive impairment with limited to no verbal communication undergoing dental treatment with midazolam conscious sedation.
Method
Bispectral monitoring was applied to patients with a neurodisability or a learning disability who were undergoing dental treatment under intravenous sedation, across three sites. A researcher observed whether the sensor could be applied and how the bispectral index changed during sedation and recovery.
Results
31 patients were recruited. In most cases it was possible to apply sensors before, or soon after, midazolam was titrated. A pattern of a decrease in bispectral values towards the end point, and an increase towards recovery was noted. Fluctuations in bispectral readings were seen during treatment, likely due to facial muscle movements.
Conclusion
Bispectral index monitoring could be a valuable adjunct when training dentists in sedation in special care dentistry and may be of particular use in patients with neurodisability.
Introduction
The use of intravenous (IV) sedation with midazolam in special care dentistry (SCD) has been widely accepted for decades.1 During sedation, cardiorespiratory monitoring is conducted using pulse oximetry and the patient is monitored clinically to ensure consciousness is maintained. To determine the depth of sedation, the clinician assesses the patient’s speech, responsiveness, change in facial expression, muscle tone and acceptance of treatment. Depth of sedation may be quantified using many scoring systems, including the Observer’s Assessment of Alertness / Sedation score (OAA/S),2 Ramsay scale3 and Glasgow Coma Sedation scale. Over 30 such systems have been described in the literature, but no universally accepted standard has been identified.4
The Wylie definition5 of conscious sedation states that verbal contact is maintained throughout the sedation procedure. However, many patients such as those with learning disabilities (LD) or neurodisability (ND), are not able to maintain verbal contact as a result of their disability. It may be difficult to elicit the level of consciousness for these patients and to determine when the end point of sedation is reached.
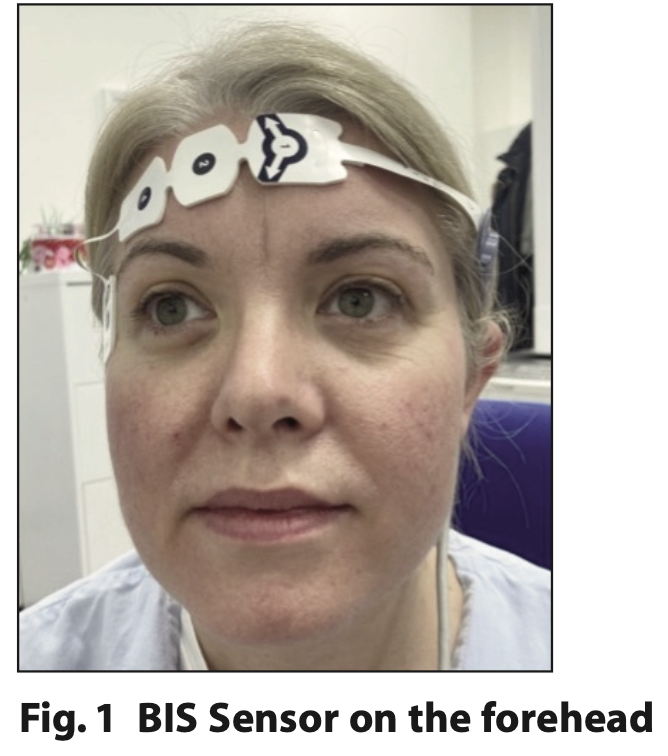
The bispectral index (BIS) monitor was developed to measure the hypnotic state (level of consciousness) during sedation and general anaesthesia. The BIS monitor works on the principles that cerebral electrical activity (as recorded in the electroencephalogram - EEG) is representative of cerebral function and that EEG waveforms change with the level of consciousness.6 A computerised algorithm converts the EEG into a numerical value ranging from 0 to 100, with zero representing no brain activity, and 100 indicative of an alert and awake status. The BIS monitor consists of a sensor, digital signal converter and a monitor screen. The sensor consists of four adhesive electrode pads placed on the patient’s forehead (Figure 1) to obtain electrical signals from the cerebral cortex.
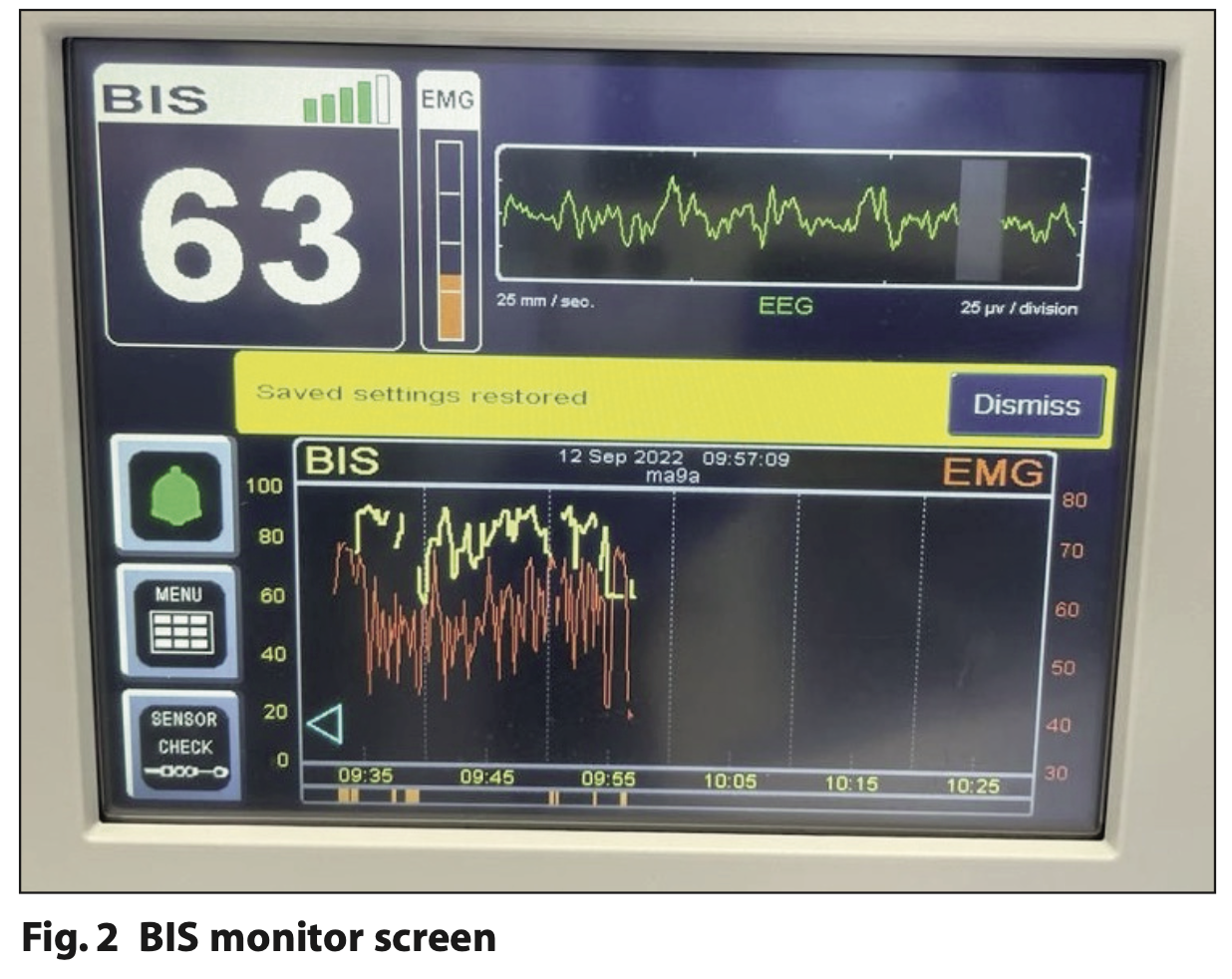
As well as the BIS value (0 to 100), the electromyogram (EMG) and signal quality index (SQI) are continually displayed on the screen. EMG is a measure of interference from the patient’s muscular activity. This is viewed on the display monitor as a horizontal orange tracing and a simplified orange vertical measure (Figure 2). SQI indicates the quality of the EEG signal and is displayed from one green column indicating poor signal quality to full signal quality with all five bars highlighted in green (Figure 2). BIS readings are displayed as a horizontal yellow tracing, and the number can be seen in white (Figure 2). BIS values of 60 to 90 have been recommended for sedation and 40 to 60 for general anaesthesia.7
Much of the research evaluating BIS monitoring for sedation has been carried out in the medical field such as in intensive care settings. There is limited research on its use in dentistry.8,9 In a study on adults with dental anxiety undergoing dental treatment under IV midazolam sedation, Shah, Manley and Craig10 found the BIS value reduced as depth of sedation increased and concluded that BIS readings were most accurate at the endpoint of sedation, where interference from muscular activity was at its lowest.
van Den Bergs11 used BIS monitoring for target-controlled midazolam infusion during lengthy dental procedures. They concluded that the device assisted in minimising midazolam dosages whilst maintaining effective anxiolysis. Munoz Garcias9 concluded BIS monitoring allowed for reduced doses of fentanyl, propofol and midazolam during implant surgery.
There is a lack of dentistry-based research on BIS monitoring for people with neurological impairments. A paper published in the Journal of Clinical Anaesthesia8 looking at participants with intellectual disability undergoing dental treatment discovered BIS monitoring reduced propofol dosages and recovery time. A non dental-based paper reported that BIS monitoring might be a ‘…useful adjunctive tool in the objective assessment of the level of consciousness in brain injured patients’.12
BIS monitoring could be useful to ascertain when non-verbal patients are appropriately sedated. However, placement of the sensor, patient movement and dental treatment may impact the reliability of such monitoring. This study investigates the feasibility of BIS monitoring in this patient cohort.
Method
Ethical approval was gained from the Greater Manchester South Research Ethics Committee. Data was collected from three locations, including hospital and primary care settings (The Royal Hospital for Neurodisability, Surrey and Sussex NHS Healthcare Trust and Kent Community Health NHS Foundation Trust). A prospective study design with convenience sampling was chosen. Patients meeting the inclusion criteria (Adults >16 years with limited to no verbal communication requiring IV sedation for their planned dental treatment) were identified during their dental assessments. A mental capacity assessment was undertaken, and best interest discussions were undertaken for dental treatment under sedation. The patients / next of kin / legally appointed advocates and / or carers were provided with verbal and written research information. Consent to participate in the study was conducted by the researchers, not the dentists providing sedation, to avoid any element of coercion to partake in the study.
Two sedation dentists and three researchers carried out the project. A calibration day was organised with four pilot cases to refine the study design and standarise data collection. The following objectives were indentified from the calibration day:
- Did the patients accept application of the BIS sensor?
- Did the BIS reading alter during sedation and in recovery?
- Did patient movement or dental treatment interfere with the sensor placement or readings?
The sedation and dental treatment proceeded as planned. The only additional process was application of the BIS sensor, which was placed after taking vital signs and pulse oximeter readings and prior to cannulation. For patients who were resistant to sensor application, the sensor was applied after administering oral, nasal or an initial titration of IV midazolam.
IV sedation and treatment were provided by an experienced consultant in SCD, with a dental nurse as the second appropriately sedation-trained person. The dentist was intentionally blinded to the BIS readings throughout the procedure. The researcher monitored the process from when the sensor was placed, the dental procedure itself, during recovery and upon discharge. A record was made of the SQI, and the researcher indicated to the dentist ‘one, two, three or four’ when individual electrode sensors needed reapplying if the signal was lost. Descriptive analysis was used to review the data.
Results
Over six months, 31 patients were recruited to the study. Ten patients had ND and 21 had LD, of which 24 were male and seven were female. Participant age ranged from 18 to 67 years old. 13 were graded as American Society of Anaesthesiologists (ASA) physical status classification 3 and 18 as ASA 2.13
One patient with ND became agitated during treatment so the sensor was removed; their data was included. It was not possible to place the sensor on one patient with LD; their data was not included.
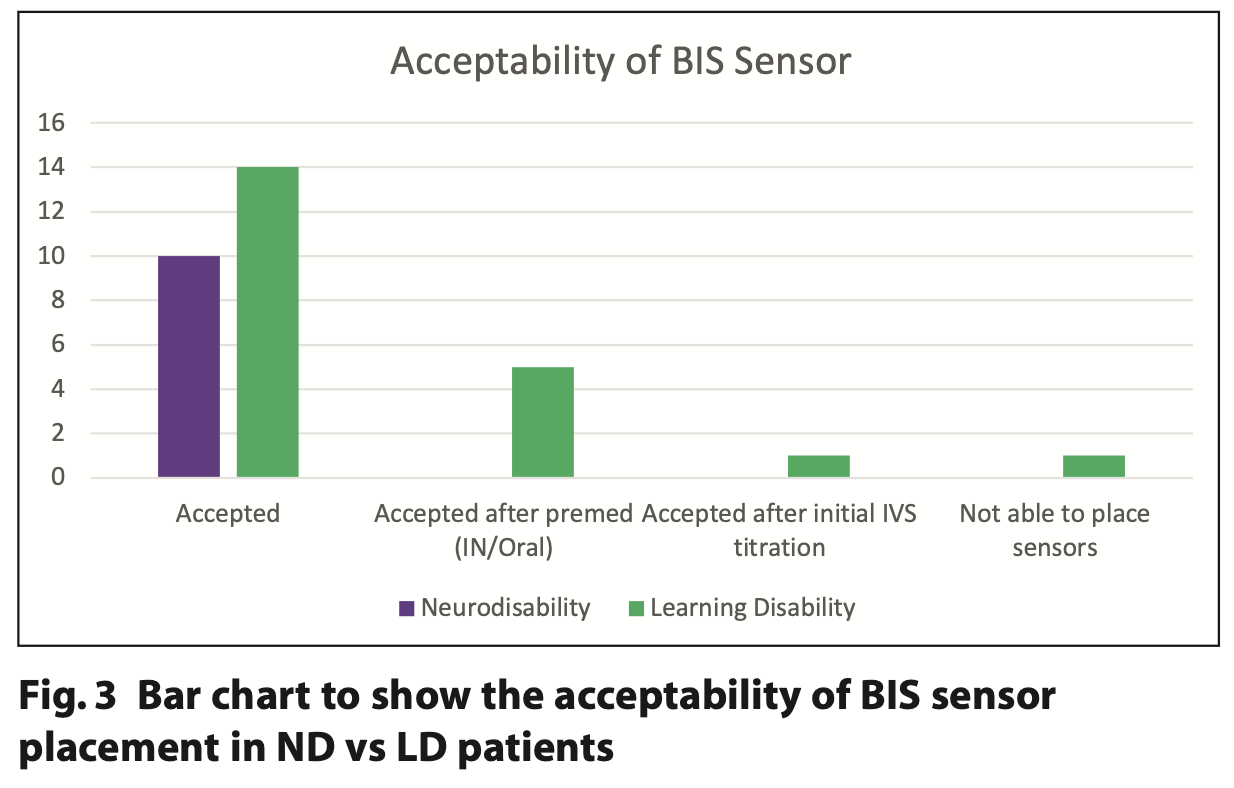
All patients with ND were able to have the sensor applied before administering sedation. Five out of 21 patients with LD accepted the BIS sensor only after having oral or nasal midazolam and one after being administered their initial 2 mg IV increment of midazolam (Figure 3).
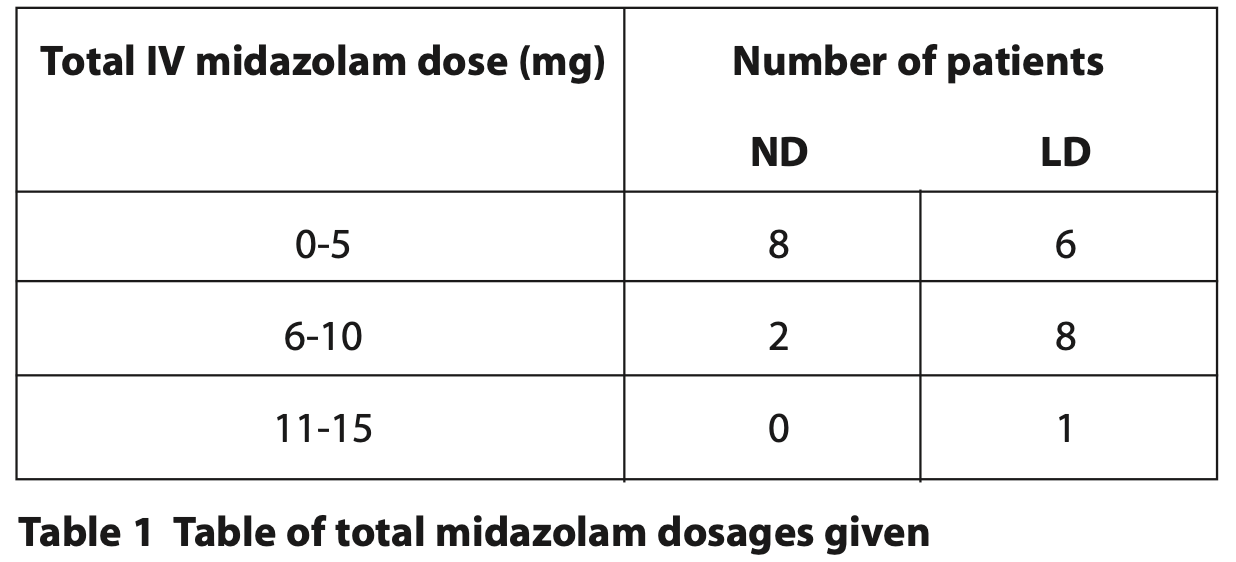
Most participants required an IV midazolam dose of between 1 to 10 mg. In general, ND patients required less IV midazolam compared to LD patients. One patient needed a total of 15 mg (Table 1).
Three patients with LD were electively reversed with flumazenil in order to facilitate safe discharge home. One patient required supplemental oxygen due to oxygen saturation dropping below 90% during treatment and recovery. Another patient became disinhibited during treatment and not all treatment was completed.
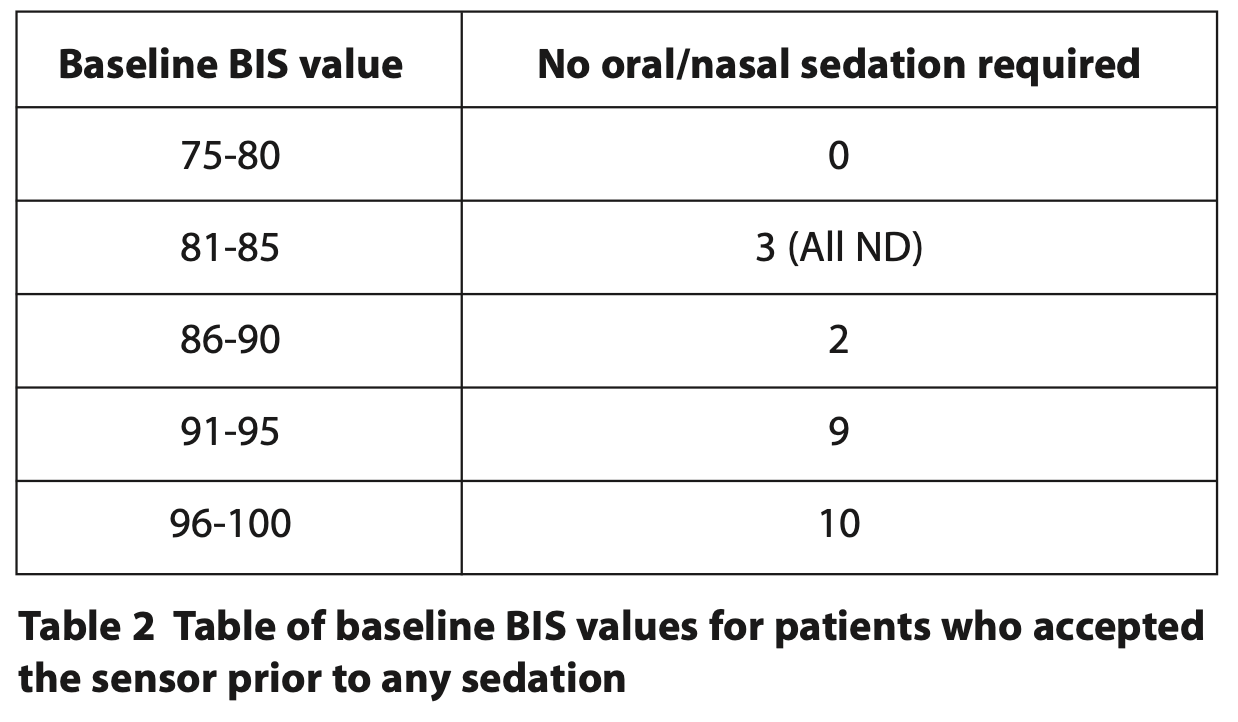
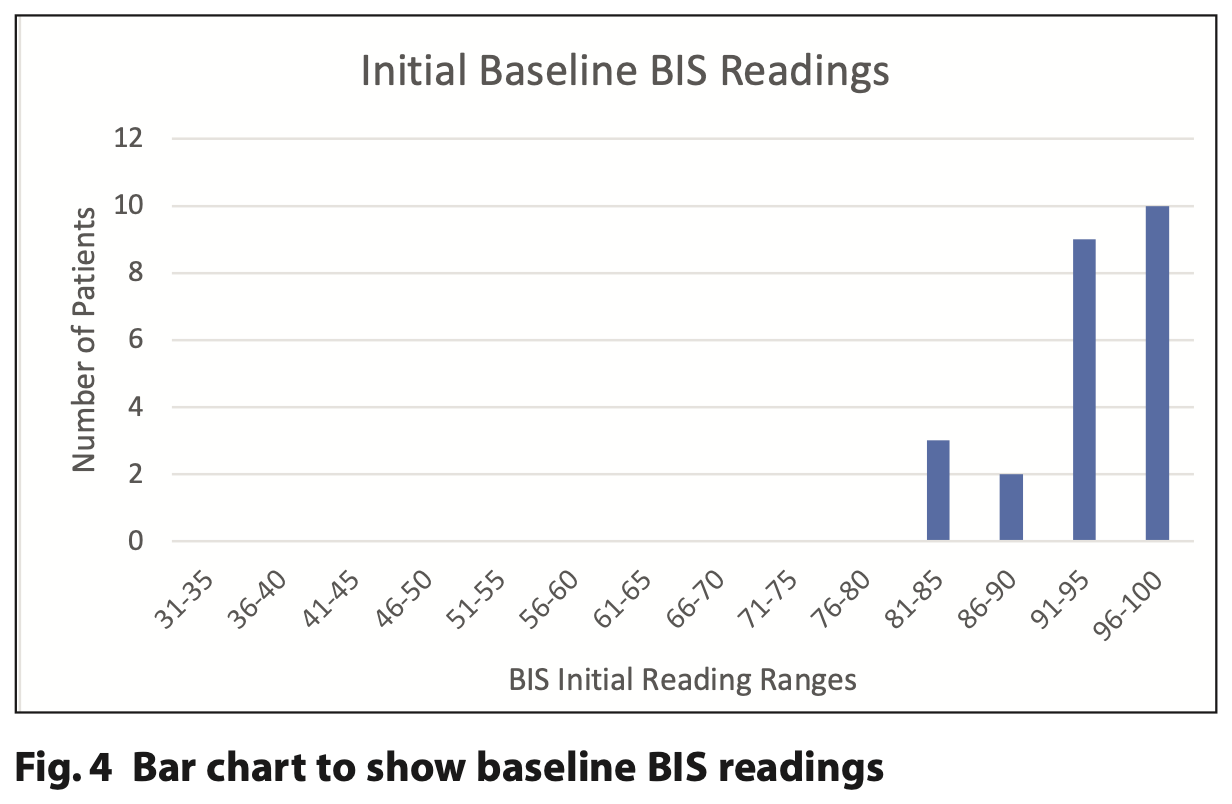
For the 24 patients who accepted the BIS sensor before any sedation, their baseline BIS values ranged from 84 to 98 (Table 2). It was noted that all three patients with lower BIS values of 81 to 85 had a ND.
For the six patients who only accepted the BIS sensor after administration of IV, oral or nasal midazolam, their baseline BIS values ranged from 78 to 98.
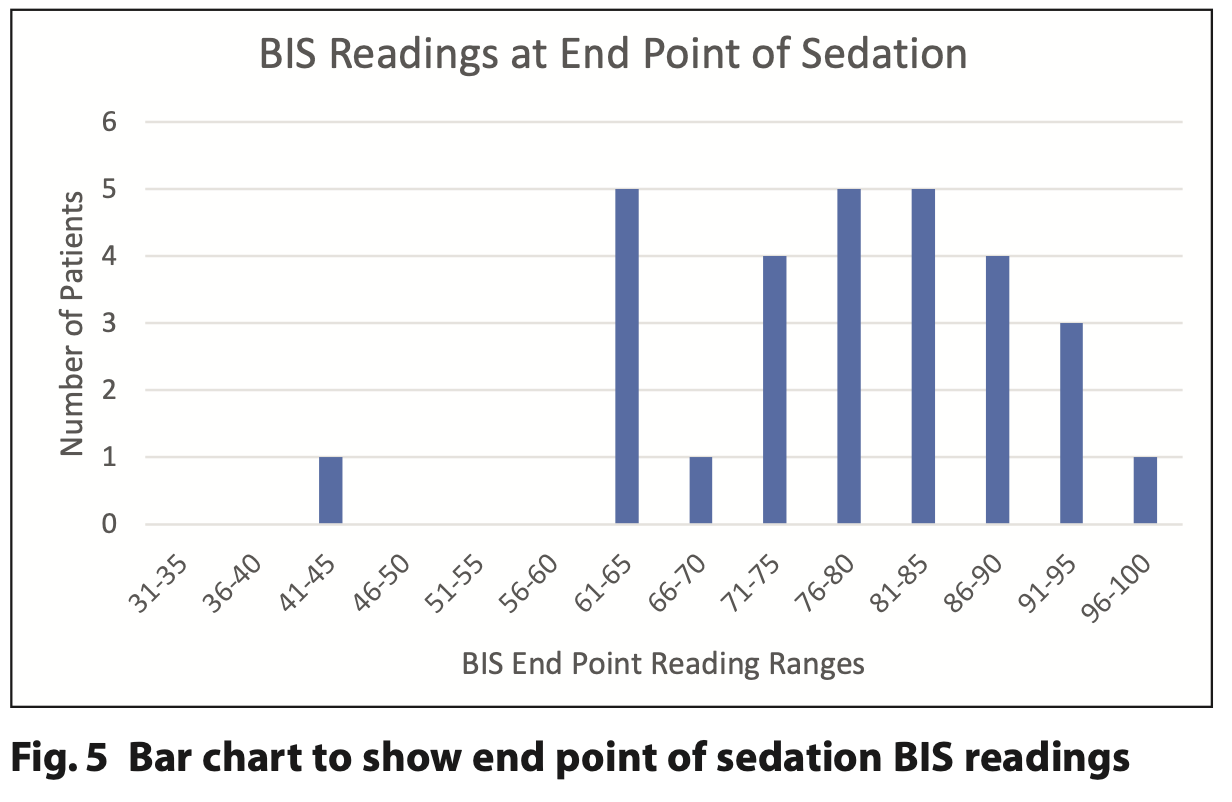
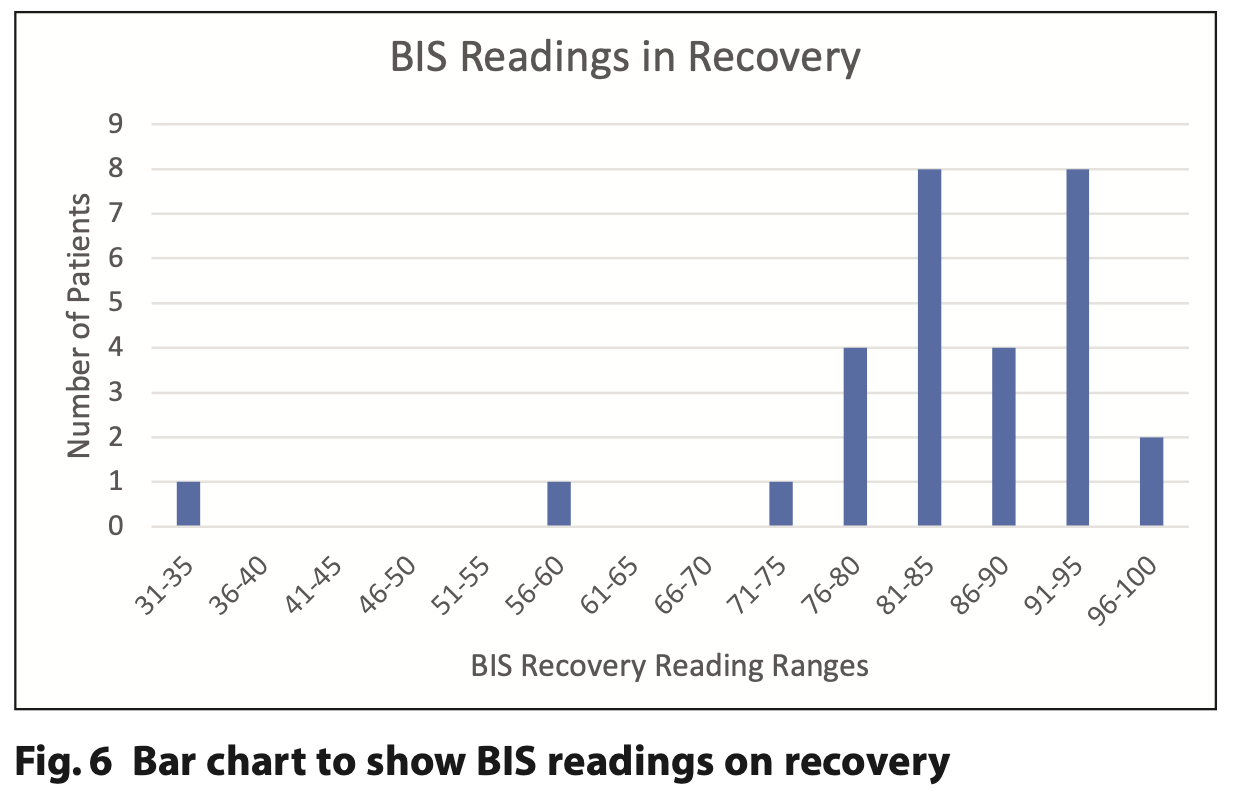
Figures 4, 5 and 6 demonstrate the initial, end point and recovery BIS readings for all study participants.
A range of dental treatment was provided including examinations, scaling, restorations, extractions and removal of failed bridgework.
While waiting for the baseline reading, 21 out of 31 patients had an initial poor signal quality. However, overall the average signal quality was 3.5 out of 5, with a mode of 4.
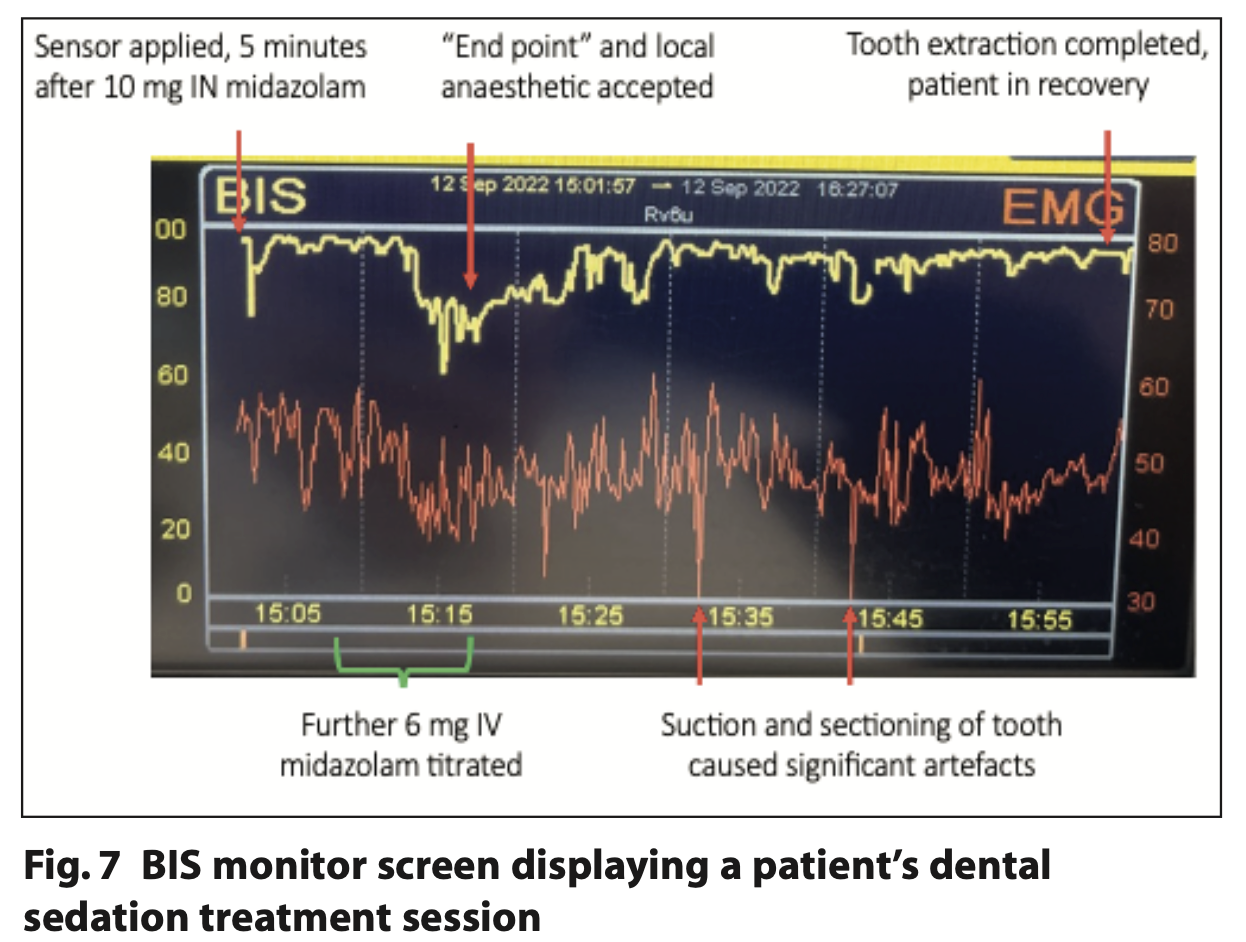
Figure 7 shows an example of a BIS trace taken during the study. It has been annotated to indicate when sedation and dental treatment was provided.
The researchers observed that BIS readings were largely unaffected by patient movements. However, muscle movement created during routine dentistry had an instant effect on BIS tracings; even placement of mouth props could elicit a marked spike in artefact and reduce signal quality. On one occasion, the sensor fell off and was reapplied but not until active treatment was completed. One case encountered a faulty sensor which had to be replaced. An ‘excessive artefact’ alert was seen throughout one case during their extractions but also on recovery when no stimulation occurred.
Discussion
The overarching aim of this project was to assess whether it is feasible to use BIS monitoring for this patient cohort. BIS monitoring can be used to indicate when a patient is sedated and can be used as an adjunctive measure when titrating midazolam to avoid potential over or under sedation.
A convenience sampling method was utilised to recruit ten cases per site to allow for some expected attrition from missed appointments. 31 cases were recruited with a variety of conditions ranging from Huntington's disease, prolonged disorders of consciousness, traumatic brain injuries, cerebral palsy, epilepsy and Angelman syndrome. As a result, generalisations between participants have limited value.
From a technical perspective, the sensor was quick to place, although it often required adjustment to achieve a baseline reading. Once successfully applied, SQI was generally good with an average score of 3.5 out of 5. However, in one case, it took 15 minutes before a baseline reading could be achieved. There was difficulty on placement of the electrodes on patients with anatomical differences eg those having undergone a craniotomy resulting in deformation of the frontotemporal region. Lower hairlines, particularly hirsute patients or those with excessive perspiration posed more of a challenge for sensor attachment and subsequent signal quality.
The display unit is a compact size (similar to a benchtop pulse oximeter), but when combined with standard sedation equipment, additional cabling did pose a potential trip hazard and hinder space within the surgery (Figure 8). With this in mind, it is unlikely to offer much benefit for the average general practice sedation patient since clinical monitoring alone should suffice. The dental team commented that clipping back the wires to avoid them moving across the patient's face during treatment was helpful.

100% of the ND group accepted initial placement of the sensor, whereas six of the 21 LD cases did not tolerate placement until some form of sedation was administered (Figure 4). The patients with ND had an overall lower level of consciousness, with reduced mobility and were physically less able to interfere with the sensor compared to the LD cohort. This is similar to placing a pulse oximeter: for many patients with care-resistant behaviours, they will not allow placement of a finger probe until midazolam has been administered.
BIS tracings appeared largely to follow a pattern similar to neurologically intact individuals.10 Figures 4, 5 and 6 demonstrate how initial BIS readings were on the higher (more alert) end of the scale (80 to 90s), then at the endpoint of sedation tended to decrease towards the 60 to 80s. On recovery the majority returned to BIS values similar to their baseline reading. Those with the lowest baseline values of 85 and below were all from the ND cohort (Table 2). This trend was also supported by Dahaba’s case of a patient undergoing dental treatment in a permanent vegetative state with a baseline BIS reading between 70 and 80, indicative of neurological damage.14 It is noted that the readings from one patient with ND are an outlier with initial readings of 65 that reduced to 45 at the end point and 35 on recovery. This patient has a history of severe traumatic brain injury with a prolonged disorder of consciousness that is a likely cause of the lower BIS readings. The patient required supplemental oxygen during treatment to address oxygen desaturation and required an additional period of monitoring until they were clinically back to their baseline. BIS monitoring, in this case, supported the need for additional monitoring of this patient post-procedure.
Considerable spikes in BIS value were observed in almost all cases upon any form of stimulation, such as the placement of mouth props, ultrasonic scaling, local anaesthetic or mirror examination. This is demonstrated in Figure 8 where a surgical extraction was carried out and BIS and EMG tracings are erratic. BIS monitoring appeared more valuable when comparing pre- and post-operative numbers upon recovery rather than as an objective measure during treatment, where signal interference was at its greatest. If the BIS value was viewed in isolation, this might mislead the dentist into believing the patient is in a lighter or heavier plane of sedation than in reality.
The ND group accepted the placement of the sensor and, on average, some had lower initial BIS values as well as on recovery (Table 2). Feedback from the sedation dentists included that it can be more challenging to ascertain when this group of patients is sedated as they often show little to no gross motor movement nor changes in facial expression compared to patients with LD where there are more clinical signs of sedation. For this complex group of patients, BIS monitoring could offer additional information, and could be particularly useful in training dentists sedating this group.
This study was conducted with two dentists providing sedation across three sites which may introduce operator bias, however, both have a similar training background and experience in managing special care patients under sedation. From an operational standpoint, the use of a BIS monitor may be best suited to three operators (one to perform the dental procedure, one to nurse and one to carry out patient monitoring).
All participants lacked the capacity to consent to dental treatment and the proposed research. Many of the next of kin commented positively that their family member was being included in research. Patients who lack capacity are frequently excluded from research opportunities and are therefore not represented within published data.
This study focussed on people with ND and LD but a similar approach could be used in future research into monitoring sedation for people with dementia. There have been studies observing how people with dementia have lower baseline BIS readings and how lower values are linked to post-operative delirium following sedation/anaesthesia.15,16
Conclusions
This study demonstrated it was possible to apply the sensor and get a reading in most patients. Dental treatment caused a lot of muscular activity, which may lead to erratic BIS values impacting its reliability. BIS readings were useful at the start of sedation and in recovery to assess when the patient is back to their baseline. BIS monitoring could be a useful adjunct in training dentists in sedation in SCD and may be of particular use in patients with ND and low baseline cognitive function. Further research into BIS monitoring could be of particular use in disorders of consciousness or locked in syndrome.
Acknowledgements
The authors would like to thank Dr Graham Manley, Loraine Macintyre, the research teams and dental teams at all sites and all the patients, families and carers who participated in this study. Thank you also to SAAD and the RCS (Eng) for the research grant for this study.
Declarations of Interest
This research was funded as part of a SAAD-FDS RCS Eng Pump Priming Grant.
References
1. Ransford N J, Manley M C G, Lewis D A, Thompson S A, Wray L J, Boyle C A, et al. Intranasal/intravenous sedation for the dental care of adults with severe disabilities: a multicentre prospective audit. Br Dent J. 2010;208: 565–9.
2. Chernik D A, Gillings D, Laine H, Hendler J, Silver J M, Davidson A B, et al. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–51.
3. Ramsay M A E, Savege T M, Simpson B R J, Goodwin R. Controlled Sedation with Alphaxalone-Alphadolone. BMJ. 1974 22;2:656–9.
4. De Jonghe B, Cook D, Appere-De-Vecchi C, Guyatt G, Meade M, Outin H. Using and understanding sedation scoring systems: a systematic review. Intensive Care Med. 2000;26:275–85.
5. The Wylie Report. Report of the Working Party on Training in Dental Anaesthesia. Br Dent J. 1981;151:385–8.
6. Rosow C, Manberg PJ. Bispectral index monitoring. Anesthesiol Clin N Am. 2001;19:947–66.
7. Johansen J W, Sebel P S, Fisher D M. Development and Clinical Application of Electroencephalographic Bispectrum Monitoring. Anesthesiology. 2000 1;93:1336–44.
8. Sakaguchi M, Higuchi H, Maeda S, Miyawaki T. Dental sedation for patients with intellectual disability: a prospective study of manual control versus Bispectral Index-guided target-controlled infusion of propofol. J Clin Anesth. 2011;23: 636–42.
9. Munoz Garcia J, Vidal Marcos A V, Restoy Lozano A, Gasco Garcia C. Utility of bispectral index monitoring during intravenous sedation in the dental office. Int J Oral Maxillofac Implants. 2012;27:375–82.
10. Shah P, Manley G, Craig D. Bispectral index (BIS) monitoring of intravenous sedation for dental treatment. SAAD Dig. 2014;30:7–11.
11. van den Berg T H, Preckel B. Bispectral Index Guided Target Controlled Midazolam Sedation: a new advanced technique for dental procedures. SAAD Dig. 2017;33: 7–12.
12. Deogaonkar A, Gupta R, DeGeorgia M, Sabharwal V, Gopakumaran B, Schubert A, et al. Bispectral Index monitoring correlates with sedation scales in brain-injured patients*: Crit Care Med. 2004;32:2403–6.
13. Doyle D J, Hendrix J M, Garmon E H. American Society of Anesthesiologists Classification. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 Sep 28]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK441940/
14. Dahaba A A. Different Conditions That Could Result in the Bispectral Index Indicating an Incorrect Hypnotic State: Anesth Analg. 2005;101:765–73.
15. Renna M, Handy J, Shah A A. Low Baseline Bispectral Index of the Electroencephalogram in Patients with Dementia: Anesth Analg. 2003;1380–5.
16. Chan M T V, Cheng B C P, Lee T M C, Gin T. BIS-guided Anesthesia Decreases Postoperative Delirium and Cognitive Decline. J Neurosurg Anesthesiol. 2013;25:33–42.